Where and When to Use Induction Chemotherapy in Head and Neck Squamous Cell Cancer
“…overall HPV prevalence in OPSCC is increasing significantly over time:
from 40.5% before 2000, to 64.3% between 2000 and 2004, and 72.2% between 2005 and 2009”
“age at OPSCC diagnosis is increasing for both HPV-positive and HPV-negative patients, and a rising proportion of older patients have HPV-positive tumors”
[[OPSCC HPV+ survival ]]
overall, HPV+ OPSCC has better outcomes than HPV- OPSCC
age matters: HPV+ pts >=70yo have similar survival to HPV- pts 50-59yo
1960s: MTX for recurrent or metastatic disease
1970s, early: MTX vs cisplatin as part of a combined approach for stage IV, M1, recurrent w/o option for salvage
- cisplatin single-agent RR 14-41% (higher if prev untx)
1970s, late: cisplatin+bleomycin, then added MTX or vinca_alkaloids, then progressed to cisplatin+5-FU
- 1977 Wayne State started a series of pilot studies, non-RCT:
- prev untx, cisplatin+vincristine+bleomycin, RR 80%, CR 29%.
- bc bleomycin had so much pulm tox and cis+5-FU (CF_PF) was the new hotness, follow-up study used CF (2 cycles, C 100mg/m2 D1, F 1000mg/m2/day x96h): RR 88%, CR 19%.
- CF regimen then changed to run over 120h (another 1g of F) and 3 cycles: RR 93%, CR 38%
- final CF regimen repeated by Radiation Therapy Oncology Group (RTOG): RR 86%, CR 38%.
- next study tried to increase cisplatin, but no benefit was shown
1980s (1979-1987):
- 5x RCT w MTX, 4/5 -ve for survival
- 1/5 +ve: gave intra-arterial MTX, benefit only present in stage II oral cavity cancer
- other RCTs done, all -ve (again other than one study, a different one, that also used intra-arterial chemo and only showed benefit in oral cavity cancer)
- noted that pts who responded well to chemo also responded well to RT
- 5x RCT w MTX, 4/5 -ve for survival
1990s: CCRT
- this paper uses CCRT to mean, specifically, “Concurrent Cisplatin-based chemoRadiotherapy”
- CCRT used adjuvantly for unfavorable surgical results (R+, extracapsular extension), definitively for unresectable disease
2000s: targeted therapy, IO, taxanes
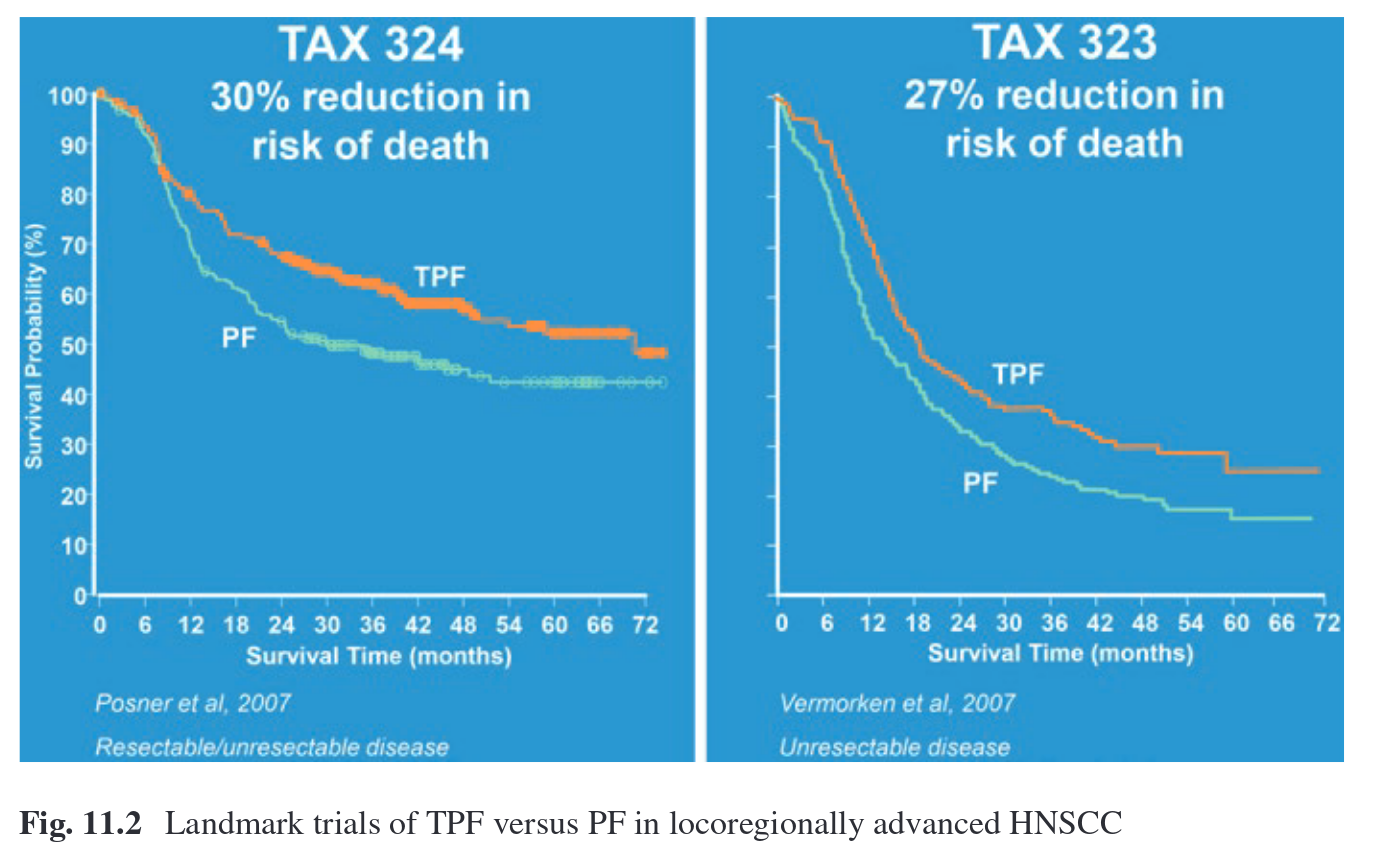
- two main RCTs, one in US and one in Europe, added docetaxel == TPF
- TPF worked better than PF (see image below), more tolerable, cost-effective.
- ~75% completed TPF+RT per protocol, ~25% had treatment delays during TPF
- did not answer whether ICT+CCRT vs CCRT alone is better, and incidences of toxicities with ICT+CCRT is higher than with CCRT alone (febrile 11%, “toxic death” up to 6%)
- European TPF (TAX323_EORTC24971):
- 3 cycles
- docetaxel 75mg/m2 x1
- cisplatin 75mg/m2 x1
- 5-FU 750mg/m2/day x5d (3750mg)
- only prev untx, unresectable LA-HNSCC
- RT afterward (4-7wks) if no disease progression, dealer’s choice RT protocol (66-74 Gy in various fractionation schema)
- Resection before or after RT, dealer’s choice and clinical situation
- American TPF (TAX324):
- 4 cycles
- docetaxel 75mg/m2 x1
- cisplatin 100mg/m2 x1
- 5-FU 1000mg/m2/day x4d (4000mg)
- prev untx, unresectable/low likelihood of surgical cure, and pts who were candidates for organ preservation
- CCRT afterward (3-8wks), 70-74Gy + carbo AUC 1.5 x7wks
- resection 6-12wk s/p CCRT if resectable disease (e.g. not CR)
- because of increased toxicities with TPF, modified regimens have been tried:
- modified TPF: Fayette J, Fontaine-Delaruelle C, Ambrun A, et al. Neoadjuvant modified TPF (docetaxel, cisplatin, fluorouracil) for patients unfit to standard TPF in locally advanced head and neck squamous cell carcinoma: a study of 48 patients. Oncotarget. 2016;7:37297–304.
- weekly carbo+taxol: Herman LC, Chen L, Garnett A, et al. Comparison of carboplatin-paclitaxel to docetaxelcisplatin-5-fluorouracil induction chemotherapy followed by concurrent chemoradiation for locally advanced head and neck cancer. Oral Oncol. 2014;50:52–8.
- TPEx (cisplatin, docetaxel, cetuximab): Zenda S, Ota Y, Kiyota N, et al. A multicenter phase II trial of docetaxel, cisplatin, and cetuximab (TPEx) followed by cetuximab and concurrent radiotherapy for patients with local advanced squamous cell carcinoma of the head and neck (CSPOR HN01: ECRIPS study). Front. Oncologia. 2019;9:6.
larynx preservation - treatment intensification (?)
- borderline resectable or unresectable oral cavity cancer
- selection tool for RT dose de-escalation in HPV+ OPSCC
- oligometastatic disease